Shockwave Is An Attractive Medical Device Firm

One of the biggest health challenges the world faces is high cholesterol. An incredible 200 million people worldwide take statin drugs daily to lower their cholesterol count.
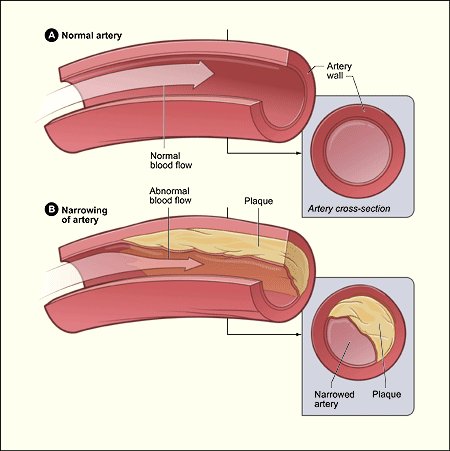
Why is cholesterol such a major focus of doctors? Because high cholesterol is believed to be a major contributor to atherosclerosis, a hardening of blood vessels caused by calcium deposits that collect on the vessel walls. This can lead to high blood pressure and, in very serious cases, complete blockages leading to heart attack and stroke.
Prevention of atherosclerosis is important, but what about when the condition has already manifested? 78% of adults over age 70 have some degree of vessel calcification. Treating the condition is important in avoiding these medical emergencies.
Traditionally, there have been two approaches. Angioplasty is the technique of inserting a catheter into a blocked vessel, using it to inflate a small balloon to open it up and then inserting a stent, which keeps it open, improving blood flow. For more severe cases, a process known as atherectomy is used, which involves inserting a small drill-like device into the vessel to loosen the plaque.
There are challenges and risks to each of these treatments. For one, angioplasty is not applicable for many cases, particularly those where there is severe blockage. Atherectomy also carries the risk of scoring off pieces of plaque that are too large and can become lodged deeper down the vessel, or even cutting too deep and puncturing the wall of the vessel itself.
It is with these challenges in mind that we arrive at today's Green Screen stock review: Shockwave Medical (SWAV). Let's have a look at this company's novel solution for atherosclerosis treatment and determine if it looks like an attractive investment candidate or not.
Re-Purposing A Familiar Treatment
Lithotripsy is a non-invasive treatment based on using sonic shock waves. It was first used in the 1980's and its primary focus up until recently was breaking up kidney stones so they can be passed or removed.
Most people have experienced how a sound-based shock wave can cause physical disruption. Think of how thunder can knock items off of a shelf, or how a low flying helicopter can cause windows to rattle. Lithotripsy is the same basic concept, just at a much smaller and more focused scale to break up organic matter within the body.
Lithotripsy has proven to be a highly successful method of treatment. Between 70-90% of kidney stone treatments are successful. It is also safe - sonic waves do not damage delicate tissues.
Shockwave Medical has simply re-purposed lithotripsy to be used in treating plaque build-up in blood vessels - what they call Intravascular Lithotripsy, or IVL.
An IVL procedure is pretty similar to a typical angioplasty. A thin, flexible tube called a catheter is inserted into the patient's blood vessel. An integrated balloon is then inflated with fluid, and electrical pulses from the catheter vaporize the fluid, generating sonic waves that loosen and dislodge the plaque on the vessel wall. The plaque can then be removed or passed through the bloodstream and the artery widened with a stent (if necessary).
Shockwave sells these catheters and the associated electronics for treating two conditions. PAD (peripheral arterial disease) is blockage of vessels in the arms and legs, while the more serious CAD (coronary arterial disease) is blockage of arteries supplying blood to the heart. CAD indications account for about 69% of sales, and PAD 31%.
Revenue Model
Virtually all of Shockwave's revenue comes from sales of the various catheters used for performing IVL in either PAD or CAD indications. Growth has been robust, with a 3 year compound annual growth rate of over 125%! This has been achieved by increasing acceptance by cardiovascular physicians given IVL's high success rate - over 90%, vs. less than 65% for the traditional treatments.
Management sees a $5.5 billion global opportunity for the PAD and CAD markets. A medium-term focus is getting IVL approved for Aortic Stenosis (AS), a condition where plaque has built up around the heart valves. The firm is in clinical development for AS treatments, but no firm timeline has been announced yet. Entering the AS market could add another $3 billion to its addressable opportunity.
Just a few months ago, Shockwave acquired Neovasc, which is developing a medical device called The Reducer to treat angina (blockage of vessels that causes pain). The device is approved in Europe and in clinical trials in the U.S. With over 800,000 angina patients across these two geographies, Neovasc presents another interesting growth avenue. Shockwave acquired the firm for what looks like a reasonable selling price of $100 million.
Altogether, we are looking at a nearly $10 billion addressable market for Shockwave Medical. Contrast that to the company's $500 million in 2022 sales, and we see the firm is only about 5% penetrated. There is plenty of room for continued 20-30% annual sales growth for many years to come.
Additionally, catheters are (obviously) disposable medical products that must be replaced after each procedure. That means that Shockwave's revenue base is basically fully recurring, with the same hospitals buying new catheters on a regular basis. That's what we like to see!
Any Moats Here?
The biggest competition for IVL are simply traditional angioplasty and atherectomy procedures. To succeed, IVL has to continue to produce better outcomes and a better safety record to justify its higher cost, both of which it has done to date.
Currently, there are no other companies offering IVL devices. Shockwave has over 50 U.S. and 70 foreign patents protecting its devices, and they have expiration dates between 2029 and 2040. This points to a REGULATORY BARRIERS moat that should hold for the next decade.
Certainly future competition is a possibility, given the significant uptake and margins Shockwave is generating. However, patents in the medical device business are harder to work around than those in the pharmaceutical space. A competitor would have to come up with something substantially novel and more effective to threaten this business. Given the fact that angioplasty has been the standard of care for over 40 years, that seems unlikely.
Management and Financials
Shockwave has a proven management team. Doug Godshall has been CEO since 2017, when Shockwave was a pre-revenue company. Since then he has successfully guided the company from $12 million in revenue in 2018 to almost $500 million last year - a mind boggling 151% compound annual growth rate!
It's hard to overstate just how difficult it is to guide a company successfully through that kind of growth ramp, but Godshall has done so. Shockwave generated its first year of substantial free cash flow in 2022, producing $113 million at a 23% margin. In Q4, it's free cash margin was over 35% vs. 24% in the prior year quarter. Given this, I think it's reasonable to assume that the company can produce free cash flow at 25-30% margins in the medium term.
The company has grown organically, too. Its only acquisition to date was the modest Neovasc deal - up until now it carried no goodwill or intangible balance, and its debt load is minimal at just 5% of equity. That has led to fantastic cash returns on capital, over 40% in 2022. I love organic growth, it is a far more efficient use of shareholder capital than large acquisitions.
Although Godshall's percentage stake of the company looks small at 1.6%, it is still worth $130 million, no chump change. Long-timer director Frederic Moll also owns 1.1%, bringing total insider ownership just over 3%. It's not a massive stake, but enough to align management's interests with our own.
Risks
I would rate Shockwave as a "medium-high" risk compared to our overall collection of stock picks.
My biggest concern is that, right now, Shockwave is a "one-trick" pony. It sells only IVL catheters and associated electronics. That means any negative occurrences in IVL could devastate the business. This includes safety incidents, superior competitive offerings, diminished doctor acceptance, etc. It also means that the company is selling into a relatively small market, which limits its long-term upside. It does look like management is attempting to broaden the product portfolio in a disciplined way, but it remains a risk at present.
Medical reimbursement is always a challenge in the healthcare industry, and one of the reasons I'm not generally a fan of investing in it. Shockwave, like most device firms, is subject to what healthcare companies and governments are willing to pay for its treatments vis-a-vis cheaper options like angioplasty. Shockwave has had some success in gaining increased reimbursements from Medicare in the U.S. and similar programs in Europe. Still, this is something to keep an eye on in the future if and when budget cuts are enacted in those countries.
Finally, CEO Doug Godshall is 57 and has been in the top seat for close to 7 years now. That's not particularly advanced age for a CEO, but still in the vicinity of where retirement could be a possibility. Fortunately, Shockwave has an heir apparent in president Isaac Zacharias, who is just 45 and with the company since 2018. Still, management transitions can be dicey, particularly in rapidly growing firms like this, and something to mention as a potential risk.
Conclusion
I'm not generally a fan of investing in the healthcare space. While there is certainly good growth potential given the advancing age (and declining health) of western countries, in healthcare there are a lot of regulatory and competitive challenges, as well as indirect payment channels that can limit revenues and margins.
Shockwave, though, has an attractive enough profile to consider for investment. Applying lithotripsy to the cardiovascular space was a genius idea, and Shockwave's early mover status gives it clear ownership. Growth potential is robust as it uses IVL's superior success and safety profile to overtake traditional treatments in PAD and CAD, and builds out a clinical solution for AS. Patents and the fact that it is the first novel option in 30+ years for atherosclerosis give me confidence in its regulatory moat. And the company's financial profile is strong and getting better.
Shockwave stock looks a little pricey to consider buying in now. But we will keep a close eye on the stock price for an attractive entry point. Shockwave Medical enters the Watch List today!
Information contained on this website represents only the opinions of the author and should not be used as the sole basis for investing decisions. By using this site, you agree to all statements in the Site Policy.
Watch List
| WDAY | -14.66% |
| VEEV | 10.18% |
| PSTG | -9.24% |
| INTU | 12.14% |
| CMG | 30.19% |
| RDDT | -3.84% |
| NTNX | 14.13% |
| CRWD | 71.52% |
| SE | 19.77% |
| SNOW | 2.45% |
| APPF | 3.69% |
Buy List
| PINS | -38.39% |
| ASML | -27.09% |
| SEMR | -42.13% |
| TSM | -45.61% |
| ZETA | -36.33% |
| GOOG | -52.81% |
| NYAX | -34.93% |
| MSFT | -33.01% |
| ODD | -33.41% |
| ASR | -30.64% |
| FLYW | -42.71% |
| HRMY | -61.01% |
| GLBE | -29.48% |
| YOU | -34.88% |
| ABNB | -36.69% |
| MELI | -27.66% |
| ADBE | -45.75% |
Hold List
| VTEX | -20.00% |
| CELH | 48.96% |
| TOST | 14.30% |
| CPNG | -19.28% |
| HIMS | -22.20% |
| PAYC | -21.97% |
| MNDY | 6.61% |
| ZS | 23.37% |
| V | -8.70% |
| ADSK | -3.90% |
| NOW | -6.74% |
| FTNT | -5.75% |
| TEAM | -11.07% |
